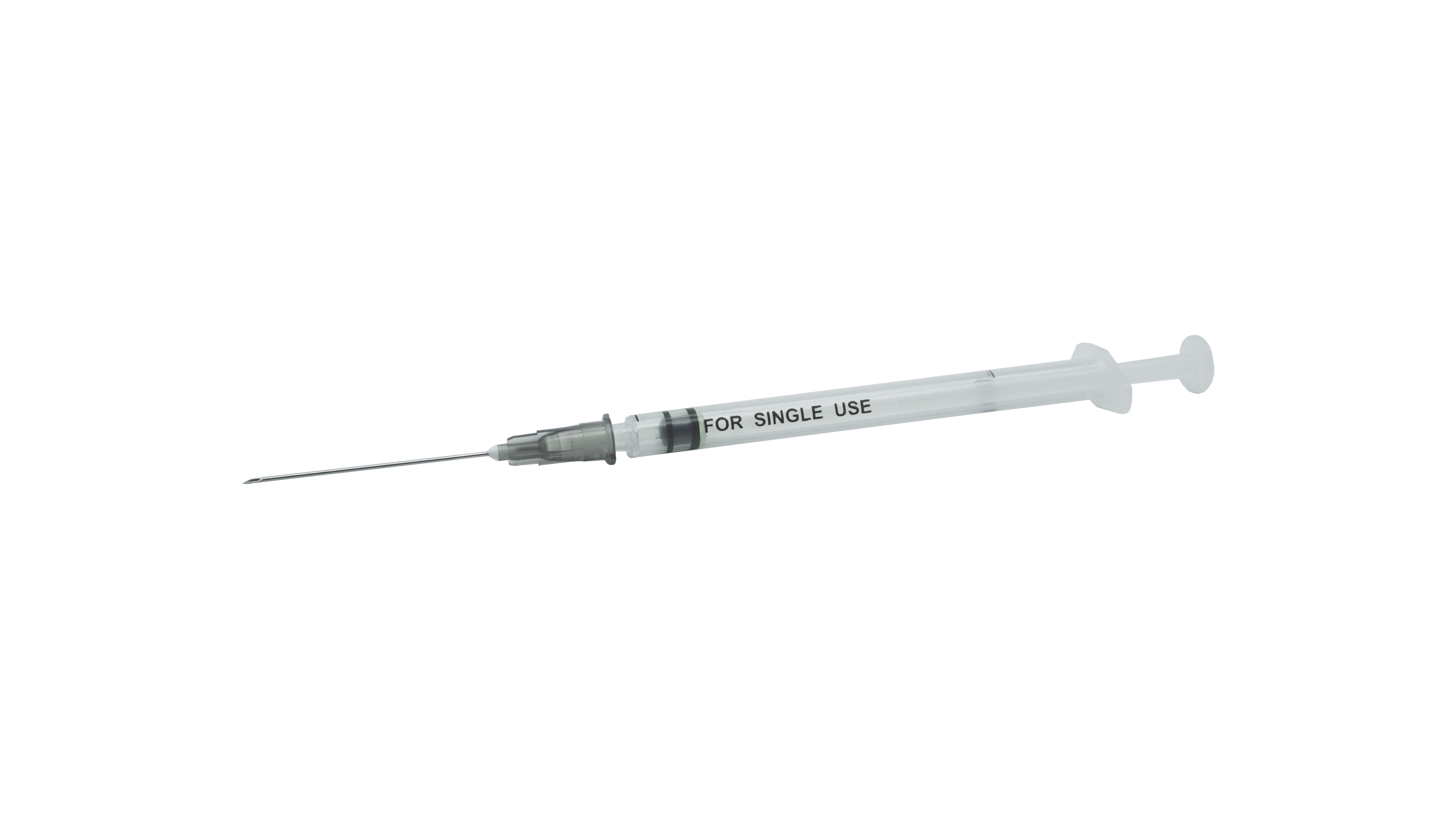
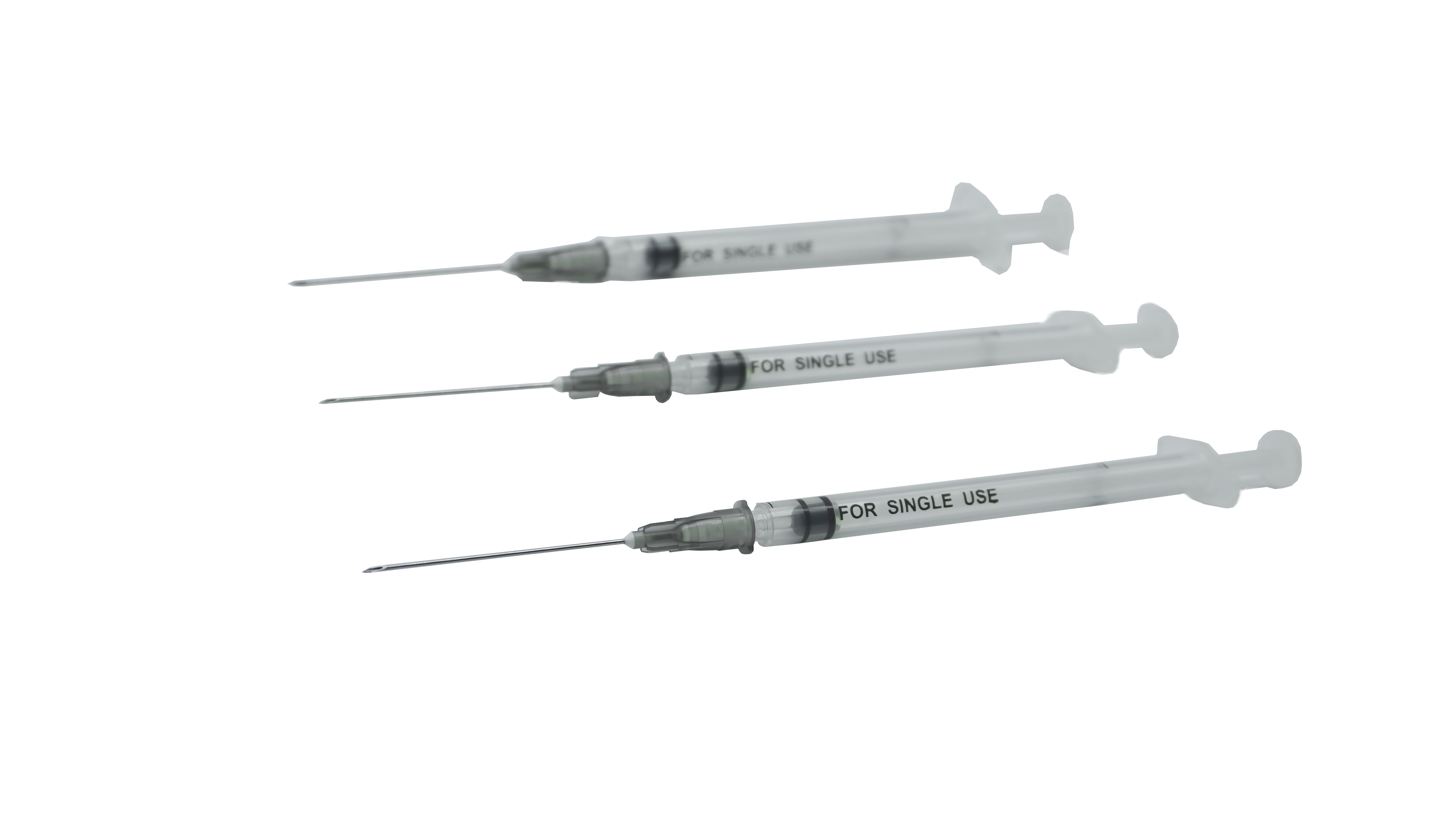
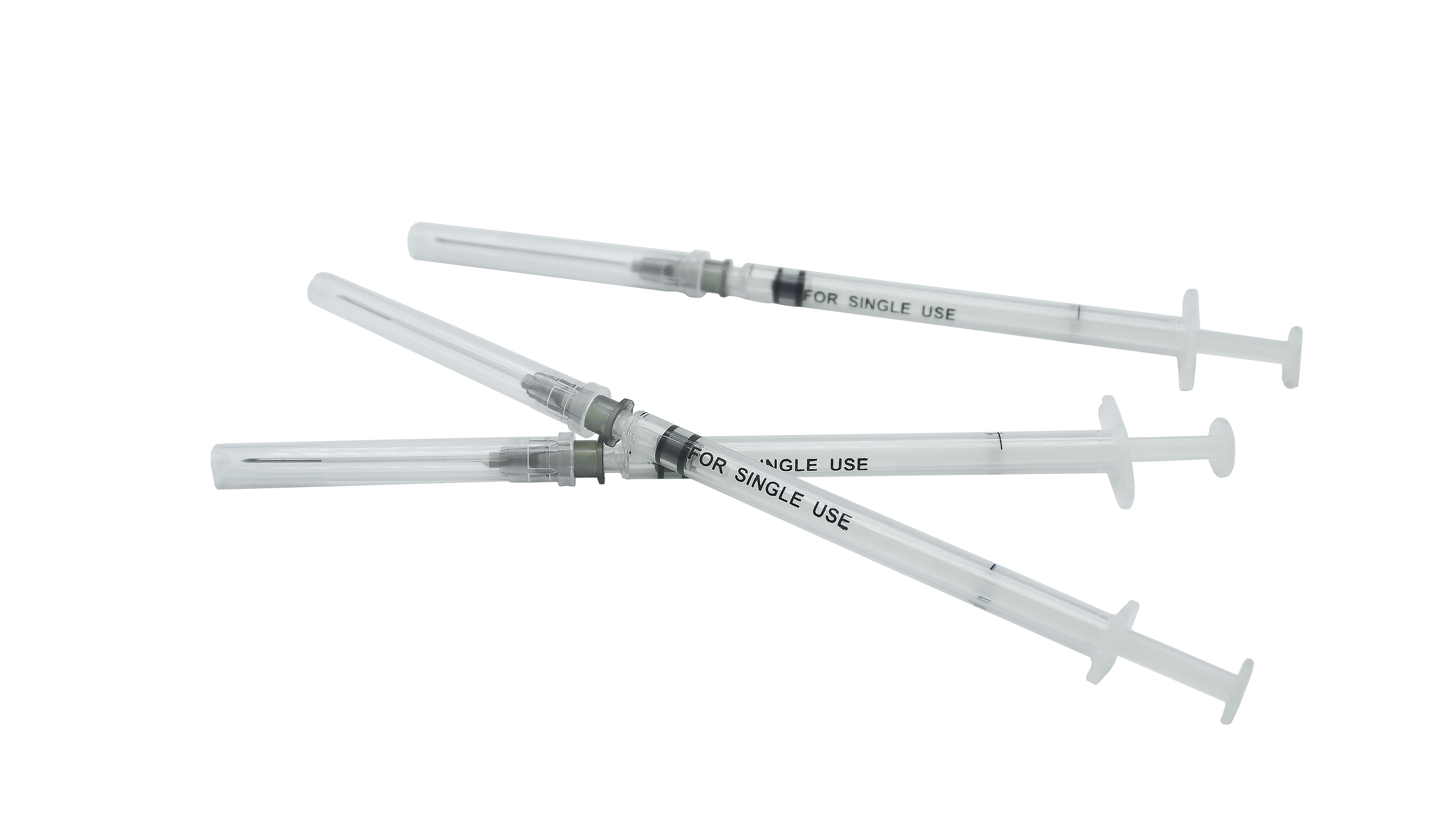
Sterile Self-Destructive Fixed-Dose Vaccine Syringe For Single-Use
Product Features
| Intended use | A single-use, self-destructing syringe indicated for immediate post-vaccination intramuscular administration. |
| Structure and compostion | The product is comprised of a barrel, a plunger, a plunger stopper, with or without a needle tube, and is sterilized via ethylene oxide for single-use. |
| Main Material | PP,IR, SUS304 |
| Shelf life | 5 years |
| Certification and Quality Assurance | In compliance with Medical Devices Directive 93/42/EEC(Class IIa)
Manufacturing process is in compliance with ISO 13485 and ISO9001 Quality System. |
Product Parameters
|
Types |
Specification |
||||
|
With needle |
Syringe |
Needle |
|||
|
0.5 ml 1 ml |
Size |
Nominal length |
Wall type |
Blade type |
|
|
0.3 |
3-50 mm (Lengths are offered in 1mm increments) |
Thin wall (TW) Regular wall (RW) |
Long blade (LB) Short blade (SB) |
||
|
0.33 |
|||||
|
0.36 |
|||||
|
0.4 |
4-50 mm (Lengths are offered in 1mm increments) |
||||
|
Without needle |
0.45 |
||||
|
0.5 |
|||||
|
0.55 |
|||||
|
0.6 |
5-50 mm (Lengths are offered in 1mm increments) |
Extra then wall (ETW) Thin wall (TW) Regular wall (RW) |
|||
|
0.7 |
|||||
Write your message here and send it to us