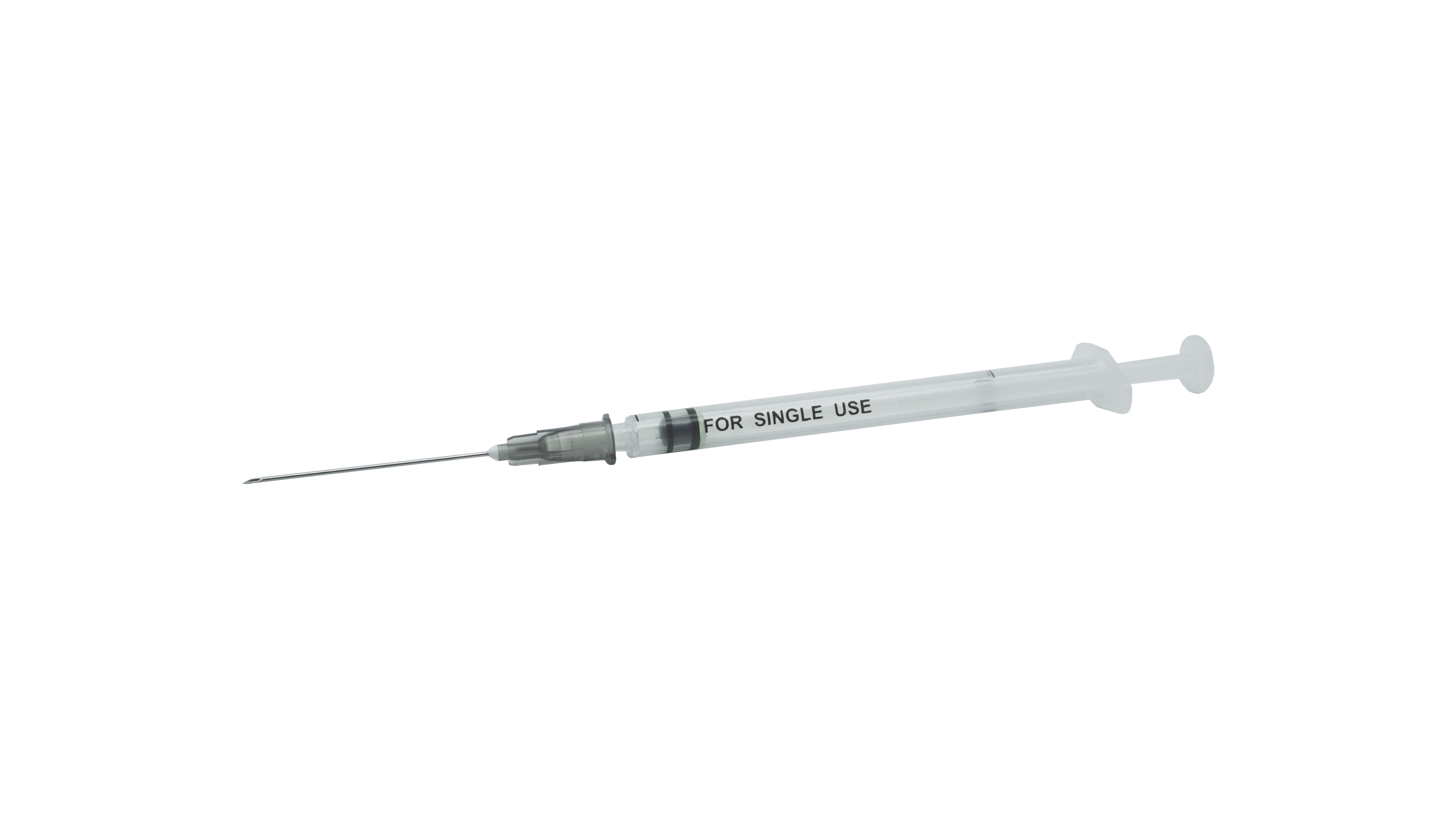
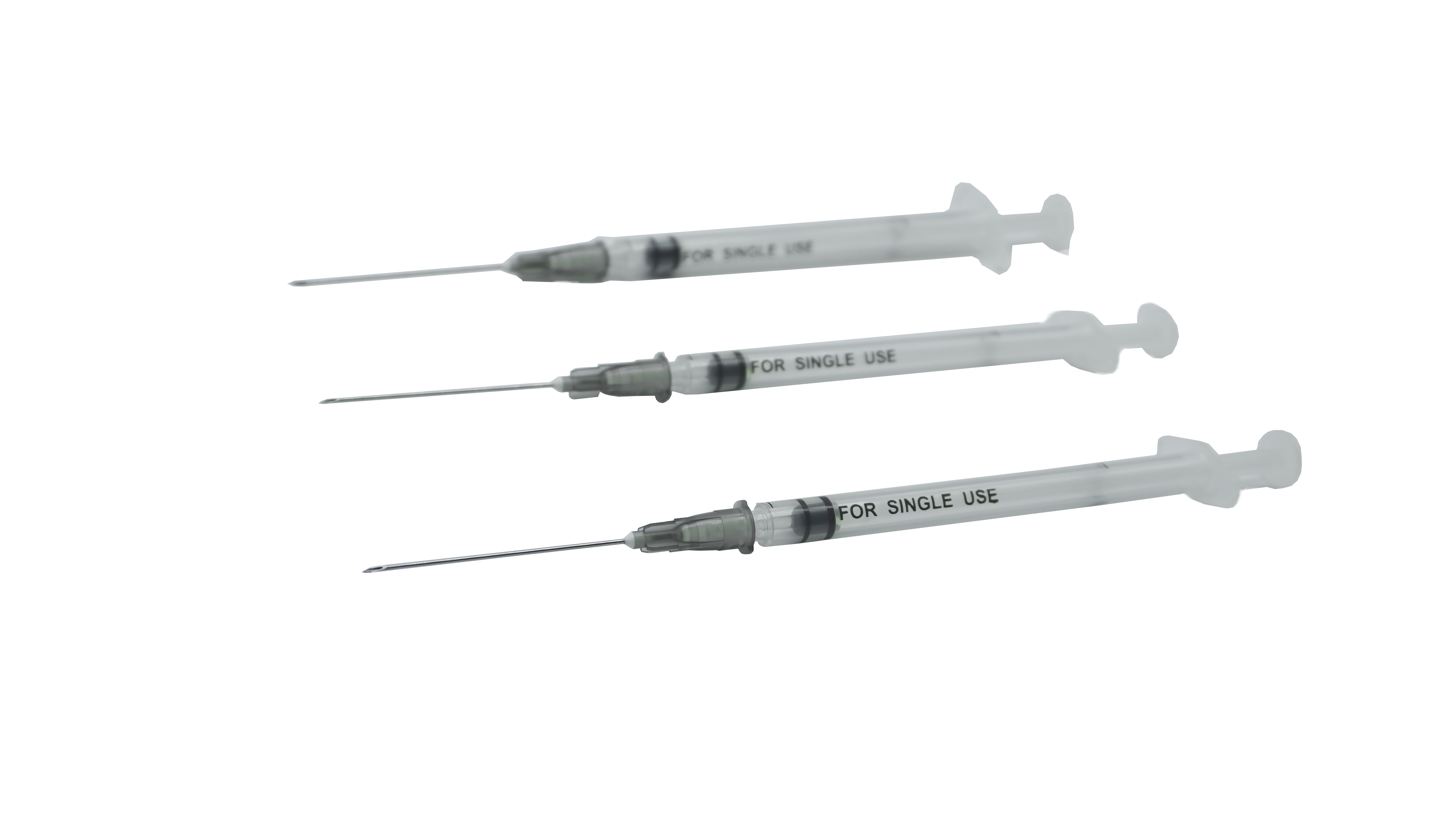
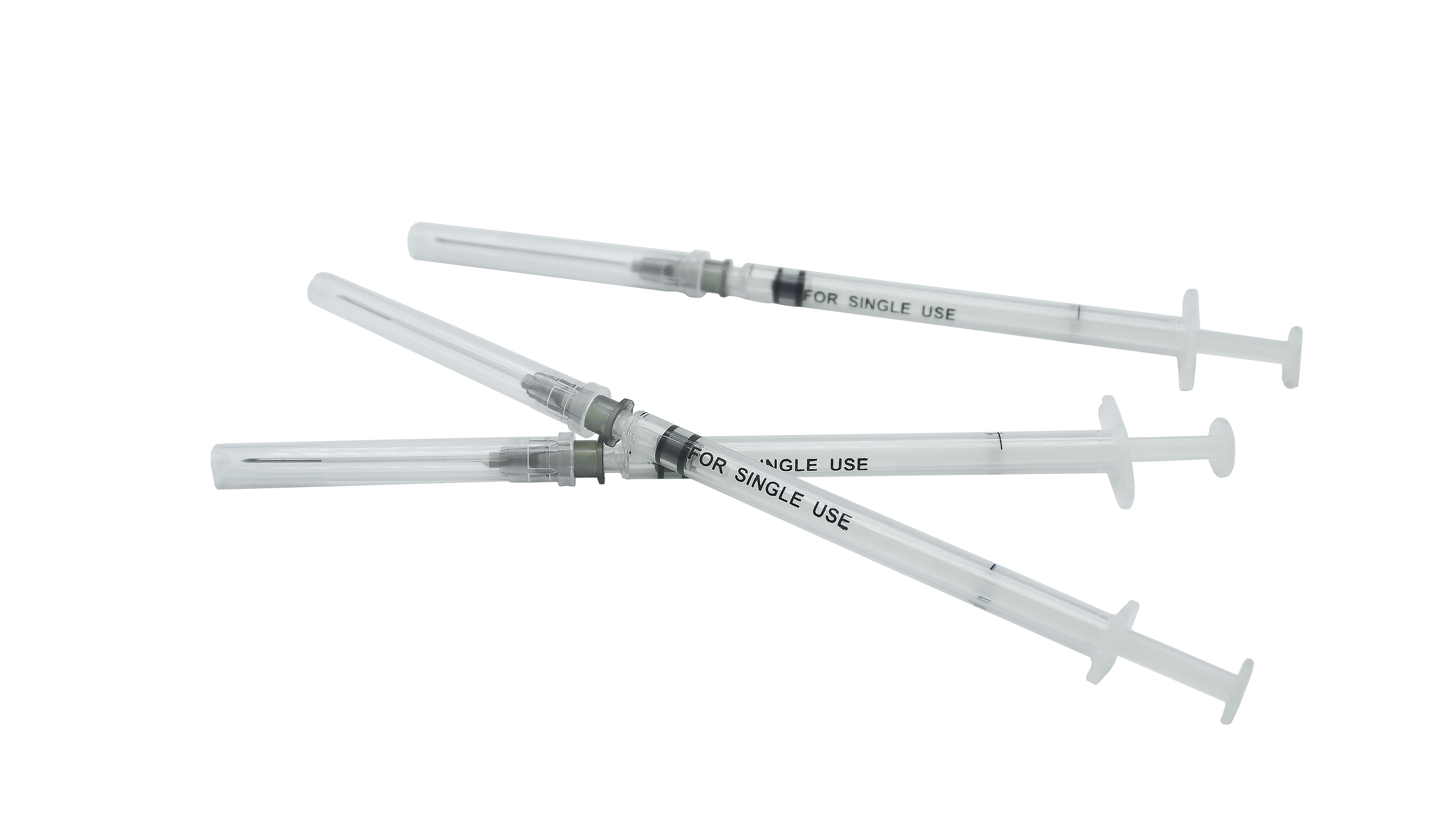
Sterile stile-stiledructive resing-dosa recorcine syringe no ka hoʻohana hoʻokahi
Nā hiʻohiʻona huahana
| Hoʻohanaʻia | ʻO kahi hoʻohana hoʻokahi-hoʻohana,ʻo ia iho i hōʻikeʻia iā Syringe i hōʻikeʻia no ka Admususculat Rort |
| Keʻano a me ka hui pūʻana | Hoʻopiliʻia ka huahana o kahi pā, he plunger, kahi mea kanu plunger, me kaʻole o kahi kīwīʻole, a he sterlilid ma Estyyne Orlings. |
| Mea nui | PP, IR, SUB304 |
| ʻO ke ola ola | 5 mau makahiki |
| Hōʻoia a me ka hōʻoia kūpono | Ma ka hoʻokōʻana i nā polokalamu lapaʻau e kuhikuhi ana i ka papa kuhikuhi 93/42 / EEC (papa inoa IIA) ʻO ke kaʻina hana hana e hoʻokō ai me ka iso 13485 a me ka'ōnaehana maikaʻi o ISO9001. |
Nā waihona huahana
| Nāʻano | Kaoaohai | ||||
| Me ka pono | Syinging | NaniAle | |||
| 0.5 ml 1 ml | Nui | ʻO ka lōʻihi | ʻAno pā | Palapala Pālolo | |
| 0.3 | 3-50 mm (Hāʻawiʻia nā lōʻihi i nā hola 1MM) | Ka paia o ka paia (tw) Pākaukau maʻamau (RW) | Long blade (lb) Pōkole pōkole (sb) | ||
| 0.33 | |||||
| 0.36 | |||||
| 0.4 | 4-50 mm (nā lōʻihi lōʻihi i hāʻawiʻia ma 1mm up) | ||||
| Me ka nele ole | 0.45 | ||||
| 0,5 | |||||
| 0,55 | |||||
| 0.6 | 5-50 mm (nā lōʻihi lōʻihi i hāʻawiʻia ma 1mm up) | 'Aʻole e pau ka pā (etw) Ka paia o ka paia (tw) Pākaukau maʻamau (RW) | |||
| 0.7 | |||||
Kākau i kāu leka uila maʻaneʻi a hoʻouna iā mākou